Abstract
Mesenchymal stromal cells (MSCs) have attracted attention as a treatment for graft vs host disease (GvHD) given their immunosuppressive and off-the-shelf properties. Although MSCs are well-tolerated in GvHD patients, therapeutic efficacy is inconsistent due to insufficient immunosuppression at inflamed tissue sites. We therefore proposed engineering MSCs with chimeric antigen receptors (CAR) to enhance immunosuppressive efficacy at sites of inflammation. In GvHD, donor T cells attack host epithelial tissues in the skin, liver, and intestinal tract in part through the interaction of T cell integrins with E-cadherin (Ecad) expressed on epithelia. We thus hypothesized that redirecting MSCs with an Ecad-targeted CAR (EcCAR-MSCs) would lead to enhanced antigen-specific immunosuppression at these sites of inflammation.
Therefore, we transduced adipose-derived MSCs with CAR containing anti-Ecad (human/canine cross-reactive) scFv with CD28ζ signaling domain generating EcCAR-MSCs at high efficiency (>90%). CD28 signal was selected for its ability to activate downstream immunosuppressive factors To test their immunosuppressive function, we cocultured EcCAR-MSCs for 24 hours with activated donor T cells with or without CAR antigen-specific soluble Ecad stimulation. Cocultures with antigen-stimulated EcCAR-MSCs led to significant T cell suppression (p=.0023) as compared to unstimulated EcCAR-MSCs and untransduced MSCs (UTD-MSCs).
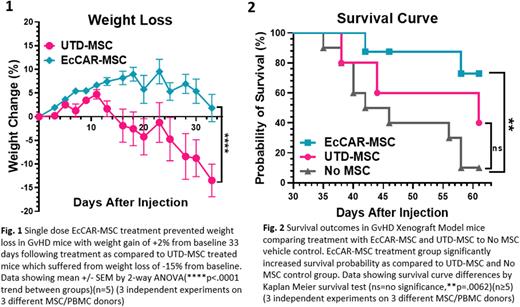
To verify enhanced immunosuppression found in vitro and test therapeutic efficacy in vivo, we tested EcCAR-MSCs in GvHD xenograft models. GvHD was induced in NOD-SCID-γ-/- mice via intravenous injection of human peripheral blood mononuclear cells (25 x 106 cells/mouse). We then treated mice with EcCAR-MSCs (1x106 cells), UTD-MSCs, or vehicle control intraperitoneally. Mice were monitored for weight loss, clinical GvHD symptom score (diarrhea, activity, posture, fur, and skin integrity), human T cell counts in peripheral blood, and survival outcomes. EcCAR-MSCs ameliorated weight loss (Fig.1), clinical GvHD score (3 vs 5.2 score, p=.0014), T cell suppression (60 vs 450cells/uL, p=.0039), and Treg induction (6.7 vs 2.1% in CD4+ cells, p=.0009), leading to improved overall survival (Fig. 2).
Mechanisms underlying superior immunosuppression in EcCAR-MSCs upon CAR stimulation were interrogated via RNA sequencing, serum cytokine assays, and immunophenotype analyses. RNA sequencing evaluated gene signatures across Ecad-stimulated and unstimulated EcCAR-MSC and UTD-MSC groups. CAR CD28-specific stimulation was supported by the upregulation of downstream immunosuppressive gene targets such as NFkB (p=.0002) and JUN (p=.0082) found only in Ecad-stimulated EcCAR-MSCs. Furthermore, gene set enrichment analysis supported TNFαactivation via NFκB (p<.0001) and IL-10 anti-inflammatory pathway (p=.036) enrichment in EcCAR-MSCs upon Ecad stimulation.
To corroborate transcriptional enrichments, serum cytokines were analyzed. Here, mice treated with stimulated EcCAR-MSCs vs UTD-MSCs revealed elevated anti-inflammatory serum cytokines, such as IL-10 (76 vs 13pg/mL, p<.0001), TNFα (35 vs 10pg/mL, p<.0001), and G-CSF (95 vs 50pg/mL, p=.0064). Immunophenotypic analyses revealed upregulated T cell inhibitory receptor expression on stimulated EcCAR-MSCs vs UTD-MSCs, including programmed death-1 (12 vs 0.7%, p=.0087) and galectin-9 (67 vs 17%, p<.0001). Collectively, our mechanistic studies support enhanced EcCAR-MSC immunosuppression through immunosuppressive gene pathway enrichment, increased anti-inflammatory cytokine secretion, and upregulated inhibitory receptors upon antigen-specific stimulation.
Finally, to demonstrate EcCAR-MSC safety, we ruled out unwanted MSC differentiation by flow cytometry (Maintenance of CD105+, CD90+, CD73+, CD34-, and CD14- stemness phenotype) and RNAseq (stem cell pathway enrichment, p<.0001) . Safety of EcCAR-MSCs were also verified in large animal models using healthy canines through intraperitoneal EcCAR-MSC injection (2x106 cells/kg), with no reported weight loss, hematological toxicity, or organ damage observed 28 days following administration. In summary, our findings position CAR-MSCs as a safe and novel therapeutic platform to enhance MSC antigen-specific immunosuppressive functionality for improved treatment outcomes in GvHD and other autoimmune diseases.
Disclosures
Sakemura:Humanigen: Patents & Royalties. Cox:Humanigen: Patents & Royalties. Kenderian:Mettaforge: Patents & Royalties; Lentigen: Research Funding; LEAH Labs: Current holder of stock options in a privately-held company, Research Funding; Kite/Gilead: Consultancy, Research Funding, Speakers Bureau; Novartis: Consultancy, Patents & Royalties: CART cell therapy , Research Funding, Speakers Bureau; Tolero: Research Funding; Viracta/Sunesis: Research Funding; Humanigen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: CART cell therapy , Research Funding, Speakers Bureau; MustangBio: Patents & Royalties; Life Engine: Current holder of stock options in a privately-held company; Morphosys: Research Funding; Juno/BMS: Consultancy, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal